FAX: (844) 270-7713 | MON-THU: 8a-5p
FDA cleared for 9 areas of the body
Nonsurgical FDA cleared fat reduction procedure, available to patients throughout Central Missouri:
Jefferson City, Columbia, Lake of the Ozarks
Females
Males

Schedule your CoolSculpting® consultation in Jefferson City, MO
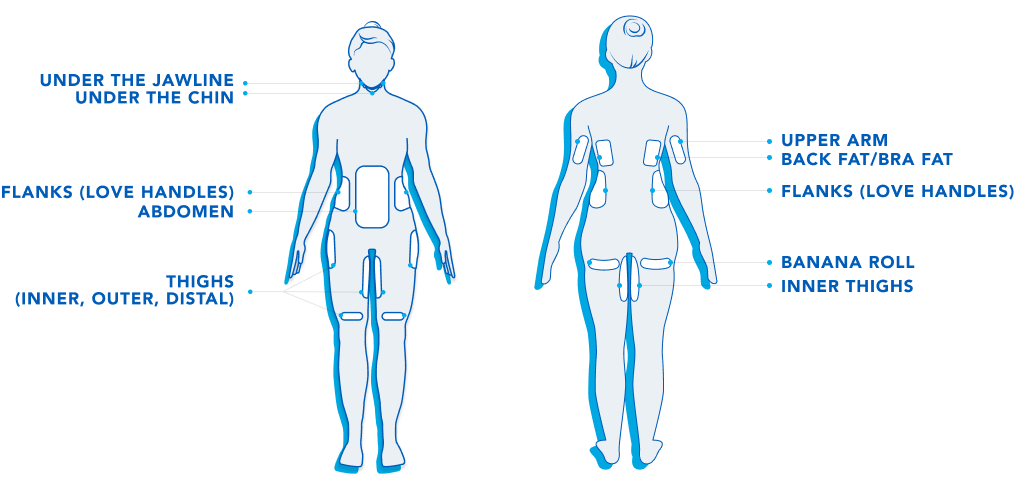
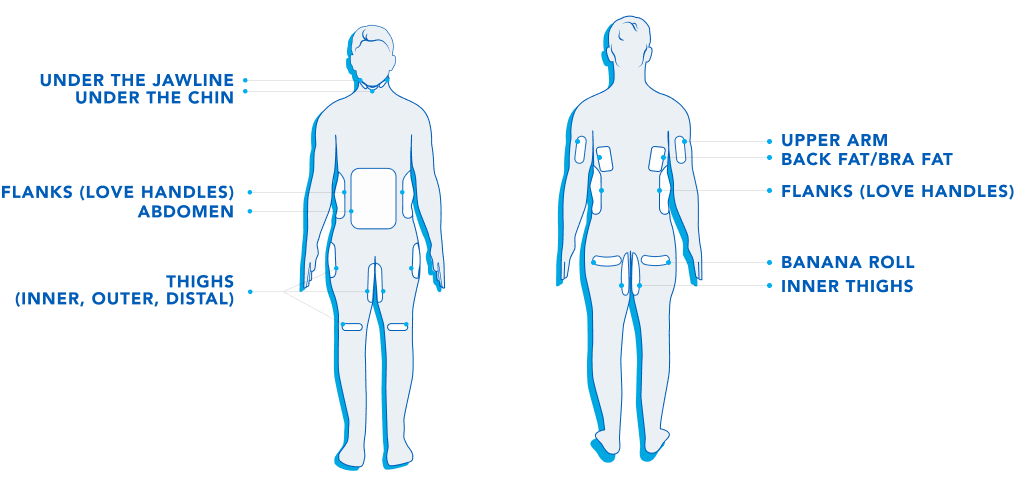
CoolSculpting® is cleared to eliminate stubborn fat under the jawline, under the chin, the upper arms, back fat, bra fat, the flank area (love handles), abdomen, thighs, and under the buttocks (banana roll).
CoolSculpting® treatments typically take as little as 35-75 minutes, depending on the area treated, with treatment sessions lasting 1-3 hours on average. For most patients, two or more treatment sessions are recommended to help reach their body contouring goals.
Schedule a Consultation
Personalized Treatment Plan
Start Treatment
USES & IMPORTANT SAFETY INFORMATION FOR COOLSCULPTING ELITE & COOLTONE
CoolSculpting® and CoolSculpting® Elite Uses
CoolSculpting® and CoolSculpting® Elite are FDA-cleared for the treatment of visible fat bulges in the submental (under the chin) and submandibular (under the jawline) areas, thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments. CoolSculpting® and CoolSculpting® Elite are not treatments for weight loss.
CoolSculpting® and CoolSculpting® Elite Important Safety Information
These procedures are not for everyone. You should not be treated with CoolSculpting® or CoolSculpting® Elite if you suffer from cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Tell your doctor if you have any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure you may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations lessen as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may happen in 1 to 10 out of 10,000 CoolSculpting® and CoolSculpting® Elite treatments (between 0.01% to 0.1%). One such rare side effect is a visible enlargement in the treated area, which may develop 2 to 5 months after treatment, will not resolve on its own, and may require surgical intervention for correction.
Please see full Important Safety Information for CoolSculpting® and CoolSculpting® Elite on CoolSculpting.com.
Patient Results May Vary.
CoolTone® Uses
The CoolTone® device is FDA-cleared for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone® is also FDA-cleared for strengthening, toning, and firming of buttocks and thighs.
CoolTone® Important Safety Information
The CoolTone® procedure is not for everyone. You should not have the CoolTone® treatment in areas with metal, electrical, or electronic implants/devices like cardiac pacemakers, implanted hearing devices, implanted defibrillators, implanted neurostimulators, drug pumps, or hearing aids.
Tell your doctor if you have any medical conditions as CoolTone® should not be used over a menstruating uterus, over areas of the skin that lack normal sensation, in patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure, pulmonary insufficiency, or pregnancy.
CoolTone® should be used with caution in patients with Graves’ disease (an autoimmune disorder that causes overactive thyroid), active bleeding disorders, or seizure disorders.
Women who are close to menstruation may find that it comes sooner, or cramping is increased or intensified with CoolTone® treatments, therefore, it is recommended to not undergo treatment during this time of the month.
CoolTone® should not be used in the heart or head areas, areas of new bone growth, over the carotid sinus nerves, or over the neck or mouth. CoolTone® should not be applied over swollen, infected, inflamed areas or skin eruptions. Caution should be used for patients with suspected or diagnosed heart problems.
Common side effects may include, but may not be limited to, muscular pain, temporary muscle spasm, temporary joint or tendon pain, and redness at or near the treatment site.
Ask your Healthcare Provider if CoolTone® is right for you.
Please see full Important Safety Information for additional information at coolsculpting.com/cooltone.
Patient Results May Vary.
The content of this Website is intended for informational purposes only and should not be used as a substitute for advice provided by a qualified healthcare professional. The Website serves as a resource platform for clinicians to assist clinics in integrating CoolSculpting® into their practice.
© 2023 AbbVie. All rights reserved. COOLSCULPTING, COOLSCULPTING ELITE, COOLTONE, and the Snowflake Design are trademarks of Zeltiq Aesthetics, Inc., an AbbVie company. Allē and its design are service marks of Allergan, Inc., an AbbVie company.
Allergan, 4410 Rosewood Drive, Pleasanton, CA 94588

